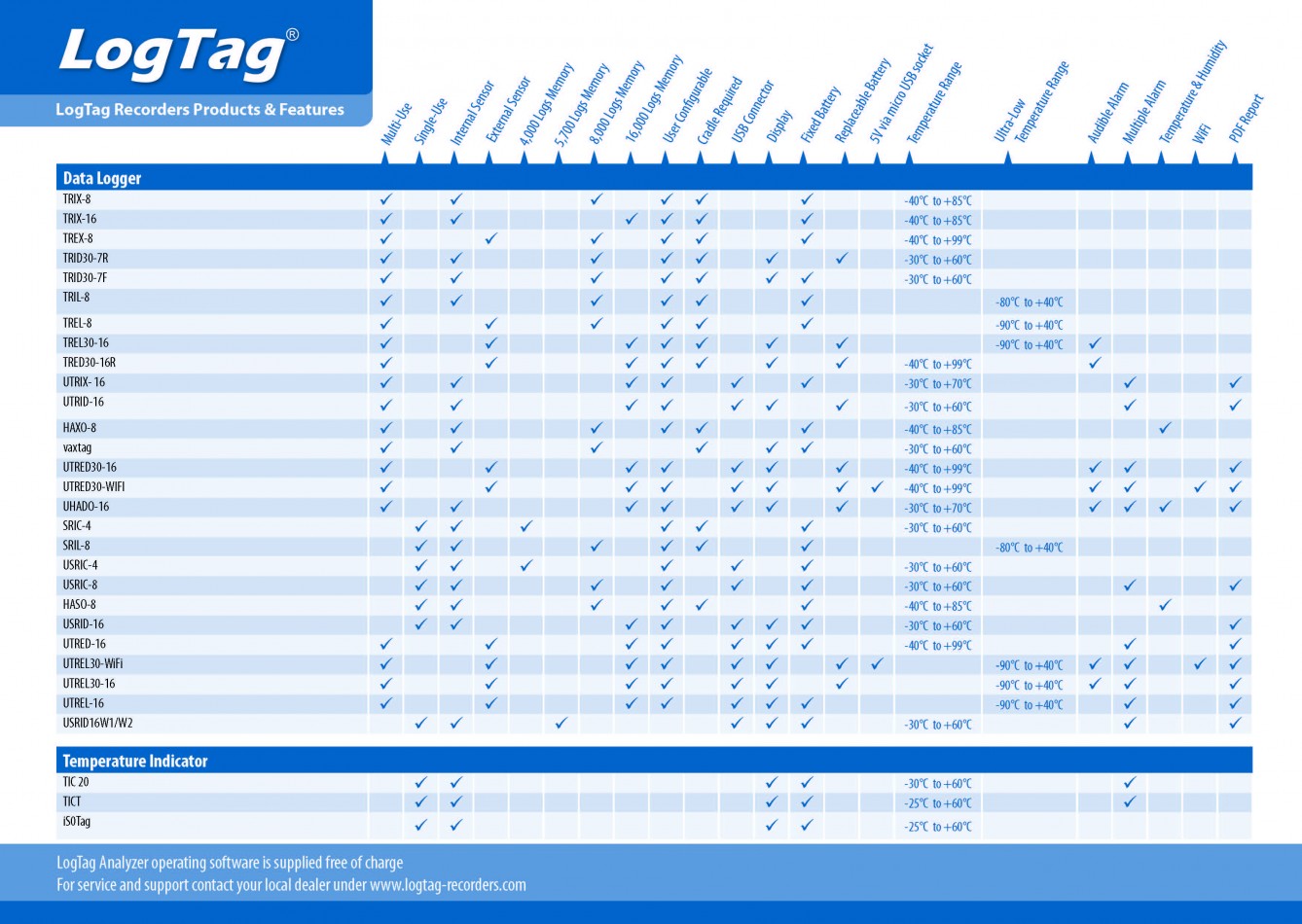
Monitoring Temperature-controlled Pharmaceutical Transports with Temperature Data Loggers
LogTag data loggers are often used in environments subject to GxP (GMP = Good Manufacturing Practice, GSP = Good Storage Practice, GDP = Good Distribution Practice). The EU guideline "Good Distribution Practice" (GDP) stipulates that pharmaceuticals must be transported according to their required storage conditions. Hence, for pharmaceutical products that are to be stored in the typical temperature ranges of +2 °C to +8 °C or +15 °C to +25 °C, temperature-controlled transport is required.
Many pharmaceutical products are temperature-sensitive and can lose their efficacy if they are exposed to excessive temperature fluctuations outside specified thresholds during transport and storage. Once the efficacy - whether caused by heat or cold - has diminished, the pharmaceutical product can no longer be saved even by subsequent correct storage. It is of the utmost importance that pharmaceutical products are handled carefully and kept in the right conditions at the right temperatures at all times. After all, the quality of pharmaceutical products is the joint responsibility of everyone, from the production to the administration of the drug.
So how can the Recipient be sure that the Medicine is still Working?
LogTag data loggers give the answer. If a compact data logger is packed together with the pharmaceutical product, the recipient can retrieve the complete measurement history including time and date and make sure that it has not lost any efficacy. The size and weight of the data logger help to ensure that there is as much room for vaccines as possible in the transport container.Alarm thresholds can be easily adapted to the different temperature requirements, and the data logger displays via its red LED if these thresholds have been breached. This is particularly important when vaccines are shipped to areas such as developing countries, where access to PCs is not necessarily a given, and a measurement protocol might not be available until some time later.
For simple monitoring of refrigerators through to regulated pharmaceutical applications where strict guidelines such as FDA 21CFR11 apply - LogTag® temperature data loggers and humidity data loggers as well as Indicators are the right choice to create a complete temperature profile!
Special features:
| Unbeatable price / performance ratio |
| THE standard in Cold-Chain transport monitoring |
| Reliable and durable |
| Hugely powerful FDA compliant software |
Choose between:
- Temperature loggers with integrated sensors or external probes
- Ultra-low temperature data loggers down to -90 °C (for example dry ice) with integrated sensors or external probes
- combined temperature and humidity data logger
- with or without display
- with integrated USB